A new case report led by UNC’s Maya Styner, MD, describes how a patient’s use of a common over-the-counter biotin supplement caused clinically misleading test results and almost resulted in an unnecessary, invasive medical procedure.

A new case report in the Journal of the Endocrine Society documents how a patient’s use of a common biotin supplement, also known as vitamin B7, caused her to have clinically misleading test results, which prompted numerous consultations and unnecessary radiographic and laboratory testing.
The patient in the case report took a 5000 mcg dose of biotin daily. Biotin supplements in that dosage are commonly sold over-the-counter, without a prescription, in many grocery and drug stores for about $8-$20 a bottle. They are marketed as being good for healthy hair, skin and nails, but there is no scientific evidence to support this claim.
In this patient’s case, “The negative clinical impact included weeks of psychological distress concerning the possibilities of hypercortisolemia or a testosterone-producing tumor. Most significantly, these abnormal test results nearly resulted in an unnecessary invasive procedure for a complex patient with a hypercoagulable state,” the case report says. Hypercortisolemia is a condition involving a prolonged excess of cortisol — a steroid hormone — in blood.
Maya Styner, MD, associate professor of endocrinology and metabolism in the department of medicine, is the case report’s corresponding author.
“The literature is lacking with regard to biotin interference with serum cortisol and testosterone immunoassays, as in our case-report,” Styner said. “Patients are ingesting supplements in a higher frequency, and higher doses, and therefore this case is timely and relevant from both a clinical and basic-science perspective.”
She added, “Our manuscript is a product of a collaboration between endocrinology, reproductive endocrinology/gynecology and clinical chemistry at UNC and at the Mayo Clinic. This collaboration enabled us to ascertain the underlying diagnosis and perform relevant research-based biotin quantification in our patient’s sample.”
Co-authors of the case report are Heather M. Stieglitz, PhD, Nichole Korpi-Steiner, PhD, Brooke Katzman, PhD and Jennifer E. Mersereau, MD. All are at UNC except for Katzman, who is co-director of the Hospital Clinical Laboratory and Point of Care, at the Mayo Clinic in Rochester, Minnesota.
In November 2017, the U.S. Food & Drug Administration issued a warning “alerting the public, health care providers, lab personnel, and lab test developers that biotin can significantly interfere with certain lab tests and cause incorrect test results which may go undetected.”
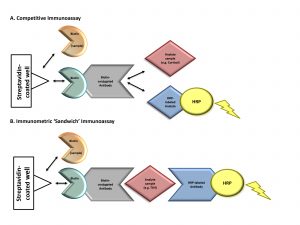
Figure 1. Mechanism of biotin interference with immunoassay methodologies. Immunoassays in this case report contain a solid phase with a wash step that physically separates the label-bound analyte/antibody from the free label. Assays that contain this step are referred to as “heterogeneous” or “multistep” (vs homogeneous assays that do not contain a wash step). (A) Competitive immunoassays are comprised of exogenous biotin-conjugated antibodies that compete for binding with an analyte of interest in the patient’s sample as well as exogenous labeled analyte. Complexes of biotin-antibody-analyte are captured to a streptavidin-coated well through strong interactions between biotin and streptavidin. A wash step removes any unbound materials. The exogenous labeled analyte is conjugated to an enzyme [e.g., horseradish peroxidase (HRP)]. Substrate is added to the well and oxidized by horseradish peroxidase, producing a luminescence signal, measured by spectrophotometry. The measured signal is inversely proportional to the concentration of analyte in the patient’s sample. Elevated concentrations of biotin in a patient’s sample can compete with biotin- antibody-(labeled) analyte complexes for binding to the streptavidin-coated well. This leads to the detection of a diminished signal causing a falsely high analyte result. (B) Immunometric “sandwich” immunoassays contain an exogenous biotin-conjugated antibody and exogenous labeled antibody. Both antibodies bind to the same analyte of interest, forming a “sandwich.” Biotin-antibody-analyte-labeled antibody complexes are captured to streptavidin-coated wells through strong interactions between biotin and streptavidin. A wash step removes any unbound materials. Exogenous labeled antibody is conjugated to an enzyme (horseradish peroxidase). Substrate is added to the well and oxidized by horseradish peroxidase, producing a luminescence signal proportional to the analyte’s concentration. Elevated biotin in a patient’s sample can compete with biotin-antibody-analyte-labeled antibody complexes for binding to the streptavidin-coated well. This leads to the detection of a diminished signal causing a falsely low analyte result. Horseradish peroxidase–labeled analyte, tracer-analyte; horseradish peroxidase–labeled antibody, tracer-antibody.