Ernest Grant, RN, PhD, FAAN president of American Nurses Association and an African American, shares why he is participating in COVID-19 vaccine trial at UNC-Chapel Hill.
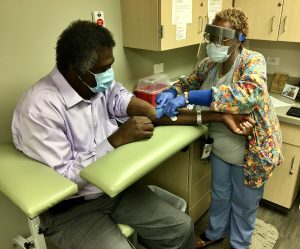
Spending a few hours at a University of North Carolina Health clinic, giving his health history and receiving an injection, might not be Ernest Grant’s favorite way to spend a Monday morning. But when he learned that UNC researchers were hosting a COVID-19 vaccine phase III clinical trial and needed volunteers, Grant didn’t hesitate to sign up.
“There’s a need for more minority participation in clinical studies like this because COVID-19 is mostly affecting black and brown populations,” says Grant. “We need to gather enough evidence of whether this vaccine will help in these populations. And if the vaccine is approved, people of color will be more inclined to try it if they know people who look like them participated safely in the trial.”
Grant, PhD, RN, FAAN, is president of the American Nurses Association (ANA) and has worked for decades as a nursing clinician and educator. He taught at the UNC School of Nursing as an adjunct faculty member until retiring from that post in 2018.(I am still adjunct faculty…I just retired from UNC hospitals) [Grant retired from UNC Hospitals in 2018, but is still adjunct faculty at the UNC School of Nursing]
“Participating in this vaccine trial is also my way of giving back to all the nurses working on the frontlines of this virus,” Grant says. “I may be removed from bedside care these days, but I want to show my support.”

COVID-19 is taking a toll on nurses’ emotional well-being, Grant knows. The ANA has surveyed nurses several times since the pandemic broke out, he says, and is seeing plenty of evidence of it toll on nurses’ mental health. “As nurses, we’re going to rise to the challenge, as we have been since COVID came to our shores,” Grant says. “But we have no way of knowing when this is going to be over, we can’t see a light at the end of the tunnel. So we’re trying to support nurses and meet their emotional and mental health needs.”
Grant urges African American and LatinX people who are wary of participating in vaccine trials to talk with someone they trust. “Talk to someone who has participated in the program who looks like you,” he says, to get a better understanding of why there’s a need to volunteer now, and why — once a vaccine is approved – you should get the injection.
“If I know that a virus like this is particularly attacking people who look like me, and there’s a vaccine out there that could save me, I would look at that a little different than just the standard mistrust of healthcare that’s out there,” he says.
In North Carolina and nationally, vaccine researchers’ efforts to recruit minority populations to participate in the clinical trials are similar to the ANA’s annual work to convince the same communities to receive flu shots, Grant says. “We find many minority communities tend to be religiously oriented, so we do a lot of meetings with congregations, answering questions they have about flu shots and holding clinics on site,” he says. “Their faith leaders are able to encourage members of their congregation to participate. If a pastor says it’s OK, it’s OK.”
Nurses also offer powerful role models in their communities. For the last 18 years, a Gallup poll has rated nursing as the most trusted and ethical profession, Grant says. “We generally get 98 percent compliance” in nurses receiving annual flu shots, he notes, and he believes most nurses will embrace the opportunity to receive a COVID vaccine as frontline healthcare workers.
On day two of participating in the vaccine trial, Grant was experiencing some of the expected side effects — chills and body aches. An app on his phone will ask him questions on how he’s feeling twice a day for two weeks. Clinical trials staff members will call him in the third and fourth week to check on him. Trials participants are followed in some form for two years.
Grant’s risk of contracting COVID-19 is less these days than it might have been when ANA business saw him flying to all 50 states and attending international meetings. Most of his meetings are virtual now from his Chapel Hill, N.C., home, but in recent months he has made a few trips to Washington, D.C., to visit ANA headquarters and the White House. No one close to him — family, friends, business associates — has contracted COVID.
“So far, I’ve been lucky,” Grant says. Knowing that the virus could kill him or at least cause prolonged illness, he looks forward to the day when a vaccine is approved — and he won’t hesitate to receive it.
To join a COVID-19 clinical trial, check out this website: https://www.coronaviruspreventionnetwork.org/