Twelve years after a five-year-old boy died from acute flaccid myelitis (AFM), researchers continued to investigate the pathology and immune response of this polio-like illness. A new case report in the New England Journal of Medicine provides evidence that Enterovirus D68 RNA and protein were found in the spinal cord of preserved autopsy tissue– proof that EV-D68 causes AFM. Dr. Matthew Vogt, MD, PhD, is the corresponding author of the paper.
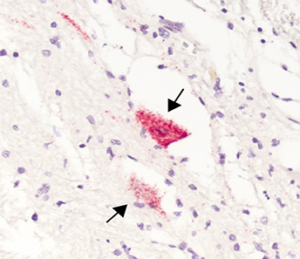
A case report published in the New England Journal of Medicine provides evidence that enterovirus D68 directly infects spinal cord neurons and that a corresponding robust immune response is present – a direct causation to the polio-like paralyzing illness, acute flaccid myelitis (AFM). Matthew Vogt, MD, PhD, assistant professor of pediatrics and microbiology & immunology at the UNC School of Medicine is the lead author of the study.
Acute Flaccid Myelitis (AFM) has emerged in recent years, with large outbreaks seen in 2014, 2016, and 2018. AFM is a serious neurologic condition that causes muscle weakness, sometimes leading to permanent paralysis, like poliomyelitis. The condition is uncommon, and it affects the nervous system, specifically the spinal cord, which causes the muscles and reflexes in the body to become weak. It can often lead to paralysis of the skeletal muscles, and in severe cases, it can affect the muscles of swallowing and breathing. Most AFM cases (more than 90%) have been in children. Recovery from AFM can vary but rarely involves full recovery of all strength. Mortality is low, with only two reported deaths out of 682 confirmed AFM cases since 2014 in the United States.
Increasing evidence has grown to show a causal link between enterovirus D68 (EV-D68) infection, one of more than 100 non-polio enteroviruses, and AFM. EV-D68 can cause mild to severe respiratory illness like runny nose, wheezing, cough, body aches, and muscle aches. But after that, AFM can develop and cause symptoms to progress rapidly. For example, it usually takes 48 to 72 hours for weakness to get to the worst point in the disease.

“Enterovirus D68 is much like the rhinovirus where it typically starts with common cold-type symptoms,” said Vogt, assistant professor in the Division of Pediatric Infectious Diseases and member of the UNC Children’s Research Institute. “Right when those symptoms start to get better, within five days or so, then weakness will begin to appear. It’s highly variable. The weakness can range from subtle to paralysis of every muscle in the body,” he said.
EV-D68 is often detected in respiratory specimens of patients with AFM, but rarely detected in their cerebrospinal fluid. Few autopsies have examined human AFM pathogenesis, so most understanding of pathogenesis is through using mouse models of infection. It was becoming known that EV-D68 was associated with AFM, but there was lacking proof of direct causation. The study began in 2019 when Dr. Vogt was a pediatric infectious diseases fellow in the lab of James Crowe, MD, at Vanderbilt University Medical Center. At that time, his research involved investigating the types of antibodies humans make in response to EV-D68 infection.
“Antibody and B cell research is very powerful now, since the techniques used identify mechanisms of immunity and at the same time can be used to isolating therapeutic molecules,” said Crowe.
“Over time scientists have done quite a bit of experimentation in the lab and found a lot of evidence showing it’s likely that EV-D68 is a major cause of AFM,” said Vogt. “Investigators have been able to use mouse models to document severe symptoms and observe the virus infecting motor neurons in the spinal cord. “Those are the exact neurons that control movement in the limbs that are now paralyzed.”
Twelve years after a five-year-old boy died from AFM in 2008, Vogt and researchers returned to this patient’s autopsy specimens to deeply investigate the pathology and immune response of the virus, especially now that the significance of EV-D68 being associated with outbreaks was further understood. The pathogenesis was confirmed when the study revealed EV-D68 directly infecting spinal cord neurons. The region of infected neurons clinically corresponded to upper limb weakness and a robust immune response was also present.
“The central nervous system, which the spinal cord is a part of, is what we often refer to as an immune privileged site, meaning that the immune system doesn’t tend to work the same in the spinal cord and in the brain as it tends to work in the rest of the body,” Vogt said. “Sometimes when there’s an immune response to an infection, it can cause lasting damage to the infected tissues, even affecting surrounding cells that are not infected. By design, cytolytic CD8+ T cells help to kill infected cells, which is a normal part of clearing viruses in most tissues. But when this happens in the central nervous system, we do not think the neurons regenerate. In the study, we found a lot of these CD8+T cells right in the area of where these infected neurons were.”
These findings further lend plausibility to the role of immunopathogenesis contributing to AFM. A final piece that connects EV-D68 causation for acute flaccid myelitis. Therefore, optimum acute treatment of AFM likely requires a multi-pronged approach focused on antiviral and anti-inflammatory strategies.
“There probably needs to be a balance,” Vogt said. “Antiviral or antibody drugs combined with an anti-inflammatory type of medicine could be good for treatment. It’s currently common for AFM patients to be treated with an immune modulating medicine, trying to limit that immune mediated damage like what the cytolytic T cells can do. The challenge is if you take away the entire immune response then you might take the brakes off that virus infection, causing the virus to go out of control. My hypothesis after seeing the data is that a patient should be treated with both options when they are available. Unfortunately, we don’t have antiviral or antibody drugs available for treating EV-D68 infection in humans right now.”
Dr. Vogt and his colleagues have used monoclonal antibodies, like what is used to treat SARS-CoV-2, to neutralize EV-D68. The data have shown when the antibody is given to infected mice during the onset of paralysis, this treatment does help improve outcomes – meaning that it will improve overall paralysis. Since this piece of evidence in the study leaves no doubt that EV-D68 causes AFM, information derived from this case report may inform treatment approaches and further direction of laboratory studies.
This research also validates the high value of conducting autopsies and biobanking tissues for cases of poorly explained infectious syndromes.
“I think what this case really shows is that by the family of this five-year-old boy electing to do an autopsy, they contributed an incredibly important observation to science that happened over a decade after their child passed away,” Vogt said. “Their child’s legacy lives on in the understanding of this disease that unfortunately took his life. Their decision could in turn be lifesaving for future children diagnosed with AFM. If we can understand how this illness works, then we can work to understand how to prevent it from happening.”
A complete list of authors in this study includes: Matthew Vogt; University of North Carolina at Chapel Hill School of Medicine, Pediatrics and Microbiology & Immunology; Peter Wright, MD; Dartmouth Hitchcock Medical Center, Sections of Infectious Disease and International Medicine, Department of Pediatrics; William Hickey, MD; Dartmouth-Hitchcock Medical Center, Pathology & Laboratory Medicine and Neurology; Tristan De Buysscher; University of North Carolina at Chapel Hill School of Medicine, Biological and Genome Sciences; Kelli Boyd, DVM, PhD; Vanderbilt University Medical Center, Pathology, Microbiology & Immunology; James Crowe, MD; Vanderbilt University Medical Center, Vanderbilt Vaccine Center.
Funding for the study was provided by National Institute of Allergy and Infectious Diseases and the Pediatric Infectious Diseases Society-St. Jude Children’s Hospital Fellowship Program in Basic Research. Dr. Vogt was supported by grant K08 AI153125 from the National Institute of Allergy and Infectious Diseases and the Pediatric Infectious Diseases Society-St. Jude Children’s Hospital Fellowship Program in Basic Research. Dr. James Crowe was supported by grant U19 AI117905 from the National Institute of Allergy and Infectious Diseases.
Media contact: Brittany Phillips