Patient Jessica Riley was referred to the UNC Wound Healing Center, part of the UNC Center for Heart and Vascular Care, for her venous leg ulcer and entered a clinical trial led by William Marston, MD
**Graphic images included**


“For want of a nail, the kingdom was lost.” This 14th century proverbial rhyme explains that small actions can result in large consequences.
Jessica Riley of Snow Camp, NC understands this all too well.
In April 2011, her legs felt itchy in the spring air, so she scratched them. A simple action that all of us have done. For Jessica, the resulting consequences resembled something from a horror film.
The small scratches on her right leg and left ankle made by her fingernails became huge wounds overnight and expanded out of control. The pain was felt to the bone, and everything made the pain worse. Movement, touch, cold weather, rainy weather.
Jessica suffers from a blood disorder which causes blood clots, leading to poor circulation in her legs and swelling in her feet. These small scratches evolved into venous leg ulcers, formed as the result of Jessica’s impaired circulation in the vein system of the legs. The ulcers were inflamed, infected, and unable to heal.
Her local physician knew immediately that Jessica needed specialized care, so she was referred to William Marston, MD, Medical Director of the Wound Healing Center in the UNC Center for Heart and Vascular Care and division chief of Vascular Surgery in the UNC School of Medicine.
Dr. Marston and his team worked diligently to heal this wound. Daily changes of dry bandages. Every other day treatments applied in a cream. Weekly visits for months and months. The wound would shrink in size, then grow again.
“It was a roller coaster ride. It seems like every week we would try something different. The wounds would downsize, then go back up. It was just up and down, up and down,” explains Jessica.
“There were days I would just sit at home and cry,” says Jessica. “I would think, ‘This is ridiculous. I’m 26. I shouldn’t have to go through this. Just take my legs off.’”
The thought of amputation was always in the back of Jessica’s mind because when Jessica was young, her grandmother had lost her leg due a blood clot that formed following hip replacement surgery. “But the physicians and nurses at UNC never once suggested amputation, which kept me going.”
By this time, Dr. Marston was serving as the UNC lead investigator in a clinical research trial that might help Jessica. “They approached me and said, ‘If we can get your wound down to a certain measurement, you will qualify for the research study,’” states Jessica.
The clinical trial was testing a topical spray containing a unique living human cell formula, called HP802-247 from Healthpoint® Biotherapeutics, which was specifically designed to treat chronic venous leg ulcers.
“So we worked hard, diligently, day in and day out. In October 2012, Dr. Marston took me out of work so I was able to rest my body, rest my legs, and get the circulation that they needed. Even though it wasn’t a high amount, it was the circulation that the wound needed.”
When the wound on her left ankle met the requirements for the clinical study, she entered the study and began weekly visits to the UNC Wound Healing Center. During week three of the study, she began receiving the spray dosing of HP802-247, not knowing if she was in the placebo group or the group receiving medication.
“I had no worries about entering the study,” states Jessica matter-of-factly. “Nothing was working, and I was willing to try anything. If you have a chance to enter a research study, do it. I had never heard of research studies like this, and I thought, ‘Why not? Let’s give it a shot. I have nothing to lose.’”
At each weekly visit, the changes in her wound were astounding. Within weeks, her wound had closed. “Though I don’t know for certain, my feeling is that I was receiving the medication because we had been treating this wound for over a year, and then in one week, I could see and feel a huge difference.”
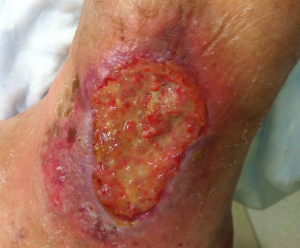
Far left: The venous leg ulcer on Jessica Riley’s left ankle and right leg were chronic wounds. A chronic wound is defined as a wound that takes longer than six weeks to heal.
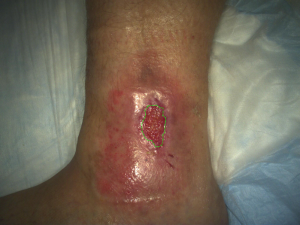
Middle: After receiving specialized care in the UNC Wound Healing Center, Riley’s open wound qualified for inclusion in an important clinical trial to test a topical spray designed to treat venous leg ulcers. Riley’s wound on week three of the study.
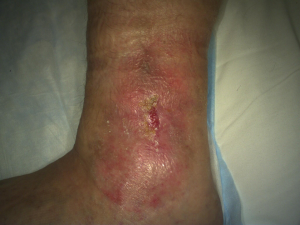
Far right: Riley’s wound on week seven of the study.
“It was very relieving to me because I thought, ‘I don’t have to deal with this anymore!’” exclaims Jessica happily. “I kept telling myself, ‘Just a few more weeks. Just a few more weeks and it will be over.’ It kept me going because I knew it was just a matter of time until it healed.”
After dealing with this wound for 18 months, Jessica laughs now and says, “Where was this research study two years ago? I have had no problems whatsoever since finishing the study. I wear my compression bandages daily to help my circulation because I don’t want this to happen again, and the wound area is still tender to touch, but there is no broken skin anymore!”
“I will be forever thankful,” says Jessica gratefully.
To view the national news coverage about Jessica’s treatment, visit these links:
Ivanhoe Broadcast News – Online News Channel
**Produced by Ivanhoe Broadcast News, Medical Breakthrough reports are distributed to network television affiliates reaching more than 80 million households every week. Click here for the full list of stations.
#####
More about the clinical research study
HP802-247 is a living human cell formula consisting of skin cells (keratinocytes and fibroblasts), which release growth factors into the wound on a cellular level for tissue regeneration, along with fibrinogen, which forms a “cellular web” for blood clotting and elasticity.
During the Phase II study, 228 patients were enrolled at 28 medical centers, including UNC. Two different cell concentrations and two separate dosing frequencies were tested with standard care, in addition to a control group, over a 12-week period.
Dr. Marston says, “In the past, some chronic venous leg ulcers were treated with skin grafts, which occasionally could break down and also required the patient to heal a partial thickness wound at the skin graft harvest site. During this study, unique living cells were sprayed on the patient’s wound, which interacted with the patient’s cells for improved wound healing.”
“In the study, we determined the best dosing of the fibroblast/keratinocyte preparation that markedly accelerated the rate of healing of the wounds.”
The Phase III trial is underway at more than 40 centers across the country and aims to enroll 440 patients.
Read more about the Phase II UNC study here.
Results of the Phase II study conducted in part at the University of North Carolina School of Medicine was published online by The Lancet.