Rahima Benhabbour, PhD, MSc, associate professor of Biomedical Engineering, led a successful effort to create an injectable implant that can release effective HIV PrEP medications into the body for six months in non-human primates.

CHAPEL HILL, N.C. – For people at high risk of contracting HIV, missing doses of their daily HIV prevention pills can have big consequences. In some cases, missing a pill can lead to lack of protection against the virus.
Since 2017, the lab of Rahima Benhabbour, PhD, MSc, associate professor in the UNC/NCSU Joint Department of Biomedical Engineering, has been working with a research team at the Centers for Disease Control and Prevention (CDC) consisting of J. Gerardo García-Lerma, MSc, PhD, Ivana Massud, PhD, and Charles Dobard, PhD and others at UNC, to develop an injectable implant that can release HIV pre-exposure prophylaxis (PrEP) medications into the body for a long period of time.
Their latest research, published in Nature Communications, shows that the team’s latest formulation can provide up to six months of full protection.
“This is the first time we showed 100% protection against multiple virus challenges in a macaque model of PrEP over an extended period of time,” said Benhabbour, “Our goal with this technology is a once or twice-yearly injection that could be self-administered.”
Daily oral PrEP has been shown to be highly effective in preventing HIV infection, but it is most effective when taken consistently. Adherence to the daily regimen can be challenging, particularly among young sub-Saharan African women because of high stigma. As a result, researchers are developing long-acting PrEP drugs and technologies that don’t have a daily burden.
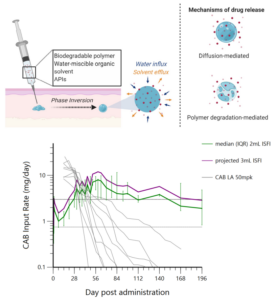
Benhabbour has been focusing on refining the formulation — the material contained in the injection. Their past research produced a formulation that consists of a solvent system, a biodegradable polymer, and the drug of interest. Cabotegravir (CAB) is an integrase inhibitor that was chosen by the researchers for the study.
Once the formulation is injected into the skin, the solvent components are absorbed by the surrounding environment. What is left behind is a solid implant of the biodegradable polymer and the drug. The drug is released via diffusion and as the polymer begins to degrade. The key aspect of this formulation is that it can slowly release the drug over time, while still having a high enough concentration to provide full protection.
Although the technology is sound, past iterations of the formulation weren’t reaching the drug plasma levels the researchers wanted. They could not reach the established benchmark for antiretroviral drugs to achieve protection against seminal HIV (SHIV) infection in macaques. So, they re-engineered the formulation, and their results exceeded expectations on multiple fronts.
The first success involved a significantly low burst release of the drug.
“The burst release was the lowest we’ve ever seen with any drug that was formulated in this injectable,” said Benhabbour. “It is important to maintain a low burst release upon injection to mitigate safety concerns due to exposure to high drug levels if the burst is too high. The low burst also allows the drug to last longer in the body, provided that initial drug levels are enough to achieve protection.”

Another benefit to this formulation is that the small implant can be removed if one needs to terminate the treatment due to any adverse reactions or a breakthrough infection. Their most recent experiments saw a rapid decrease of the drug’s levels in the plasma once the implant is removed from the body.
For Benhabbour, “the cherry on top” was the efficacy results from the macaque challenges. Macaques, which have similar immune systems to humans, were challenged with simian HIV by the rectal route. Rectal exposure is one of the most efficient routes of HIV infection.
Over the study, 6 macaques were exposed to SHIV weekly over several months. Despite a cumulative of 38 challenges, none of the macaques contracted simian HIV.
When the dosage was determined to be effective in the macaques, it was up to Mackenzie Cottrell, PharmD, MS, assistant professor in the Division of Pharmacotherapy and Experimental Therapeutics at the UNC Eshelman School of Pharmacy, to model the length of protection in humans. Her model estimated that the same dose given to the macaques would stay in the human body for 5.6 months.
The next step in their research is to adapt the technology for human use. To do so, Benhabbour and the CDC team will acquire funding to support IND-enabling studies prior to translation to human clinical trials.
“UNC is home of world class experts in clinical trials for HIV prevention,” said Benhabbour. “It would be terrific to take advantage of this in-house expertise in clinical trials to evaluate our technology.”
Media contact: Kendall Daniels, Communications Specialist, UNC Health | UNC School of Medicine