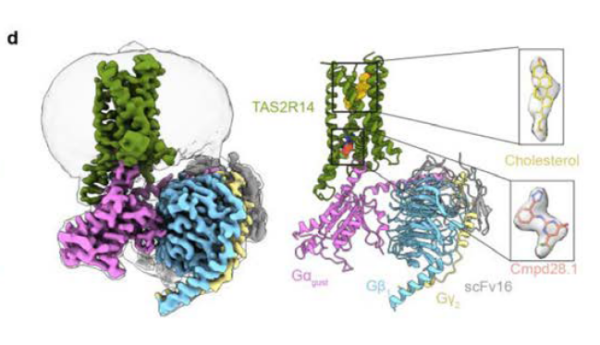
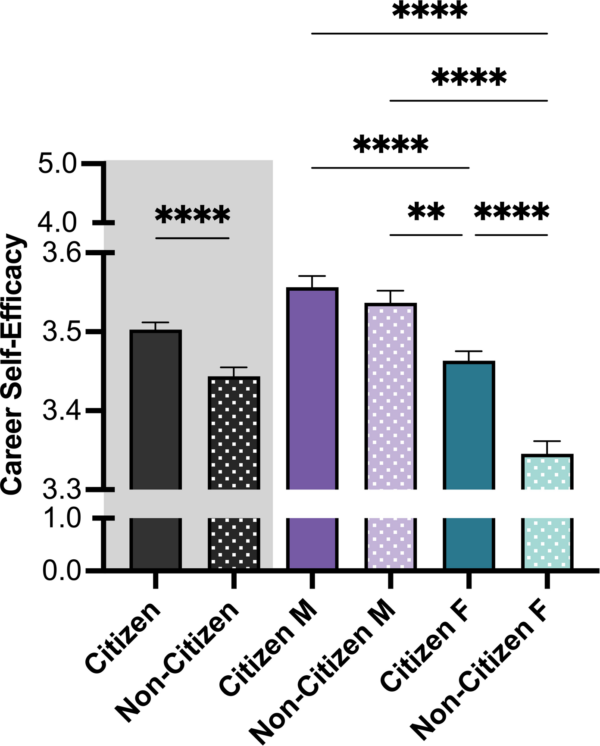
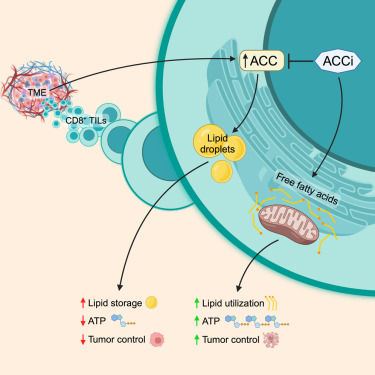
April 24, 2024
Detection of Missed Colorectal Cancer Remains Low for Most Diverticulitis Patients
Published in the Clinical Gastroenterology and Hepatology journal, a study led by Anne Peery, MD, and Walker Redd, MD in the Division of Gastroenterology and Hepatology, shows that the prevalence of colorectal cancer is low in most patients with diverticulitis. However, patients with complicated diverticulitis are the exception.